Abstract
Introduction: DNA mismatch repair (MMR) is a major mechanism cells use to repair erroneous insertion or deletion of bases during DNA replication. MMR deficiency (MMR-d), including rare germline mutations (Lynch syndrome) and somatic inactivation, has been extensively studied in solid tumors, such as colon, endometrial, pancreatic, and ovarian cancers. The prevalence of MMR-d in solid tumors ranges from 1% - 20% of somatic defects, and patients with MMR-d tumors have a longer overall survival. It is also well-known that MMR is an integral part of activation-induced cytidine deaminase (AID)-mediated somatic hypermutation and immunoglobulin class switch processes in normal mature B lymphocytes, while mice deficient in the MMR genes are prone to develop T and B cell lymphomas. It is also known that follicular lymphoma (FL) carries a high degree of somatic hypermutation with some heterogeneity induced by AID. Our understanding of MMR status in human blood cancers, especially in FL remains very limited. One could hypothesize that MMR-d status could permit cells to acquire and sustain high-degree of mutation rates, better sensitivity to DNA damaging chemotherapy while hindering rapid cell proliferation, which could manifest to a better prognosis in patients. Herein, we conducted an exploratory analysis to understand the frequency of MMR-d status and its prognostic significance in FL.
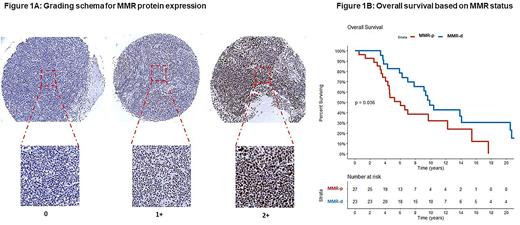
Methods: Following approval of the Institution Review Board, we assessed the expression of MMR proteins; MLH1, PMS2 and MSH6 on formalin fixed paraffin embedded (FFPE) lymph node tissue obtained from patients with FL by immunohistochemistry (IHC) using standard methods on Ventana Benchmark XT automated stainers (Ventana Medical Systems, Tucson, AZ). Dako (clone ES05), Cell Marque (clone EPR3947) and Biocare (clone BC/44) antibodies were used to stain MLH1, PMS2 and MSH6, respectively. Fifty cases of FL with existing FFPE blocks were selected randomly. The expression of MLH1, PMS2 and MSH6 proteins in the tumor cells was scored as 0, +1 or +2, (Figure 1A). Cases with absence of the IHC signal (score of 0) in any one of the three proteins were classified as MMR-d. Progression free survival at 12 months (PFS12) or 24 months (PFS24) was defined as being free from disease progression or death at 12 or 24 months, respectively. All time-to-event analyses were done from the time of diagnosis.
Results: Fifty patients with FL were included in the study. The median age at diagnosis was 65 years (range 34-85) and 58% were females. At initial diagnosis, 36 (72%) patients had advanced stage (stage 3, n=8, stage 4, n=28) disease. Of the 50 patients, 26 (52%) required treatment; chemotherapy (n=14), radiation treatment alone (n=9), surgery alone (n=3).
Out of the 50 patients, 23 (46%) were classified as MMR-d due to lack of expression of one of the three assessed proteins: MLH1 (n=1, 2%), PMS2 (n=11, 22%) and MSH6 (n=19, 38%). The patient who had MLH1 loss had concomitant PMS2 loss. Stage IV disease was observed in 52% of the MMR-d patients and 59% in the MMR proficient (MMR-p) patients, p=0.77. Median age at diagnosis was 62 (range: 34-84) years and 67 (range: 34-85) years in MMR-d and MMR-p patients, respectively, p=0.1. FLIPI score was similar between the MMR-d [low n=10 (43%), intermediate n=9 (39%), high n=4 (18%)] and MMR-p [low n=5 (18%), intermediate n=14 (52%), high n=8 (30%)] patients, p=0.16. Fourteen (61%) of MMR-d patients required initiation of therapy upon diagnosis compared to 12 (44%) of MMR-p patients, p=0.27.
The median follow-up for the entire cohort was 17 years [95% confidence interval (CI): 11.7-29.4]. The median overall survival (OS) of MMR-d patients was 10 years (95%CI: 7.8-20.7) compared to 6 years (95%CI: 4.5-NR) in MMR-p patients, p=0.036, Figure 1B. In patients with MMR-d and MMR-p, 83% and 55% achieved PFS12, p=0.04 and 65% and 41% achieved PFS24, p=0.08, respectively. After adjusting for FLIPI score, MMR status remained associated with OS (MMR-d HR: 0.51, 95%CI: 0.2-1.02, p=0.05).
Discussion and Conclusion: In this pilot study, MMR-d in the FL tumors was common (46%) and, as predicted, was associated with a favorable OS. Association of MMR-d status with PFS in general and patients with early progression (short PFS12 and PFS24) and relationship of MMR-d with types of therapy need further study in large FL datasets.
Paludo: Karyopharm: Research Funding. King: Celgene/BMS: Research Funding. Maurer: Celgene: Research Funding; Genentech: Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Nanostring: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Nowakowski: Celgene, NanoString Technologies, MorphoSys: Research Funding; Celgene, MorphoSys, Genentech, Selvita, Debiopharm Group, Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees. Witzig: Karyopharm Therapeutics, Celgene/BMS, Incyte, Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS, Acerta Pharma, Kura Oncology, Acrotech Biopharma, Karyopharm Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal